What Best Describes Trends in Electronegativity on the Periodic Table
Periodic Table Trends. Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound.

Periodic Trends In Electronegativity Ck 12 Foundation Chemistry Periodic Table Ionization Energy Teaching Chemistry
Electronegativity increases left to right and bottom to top.
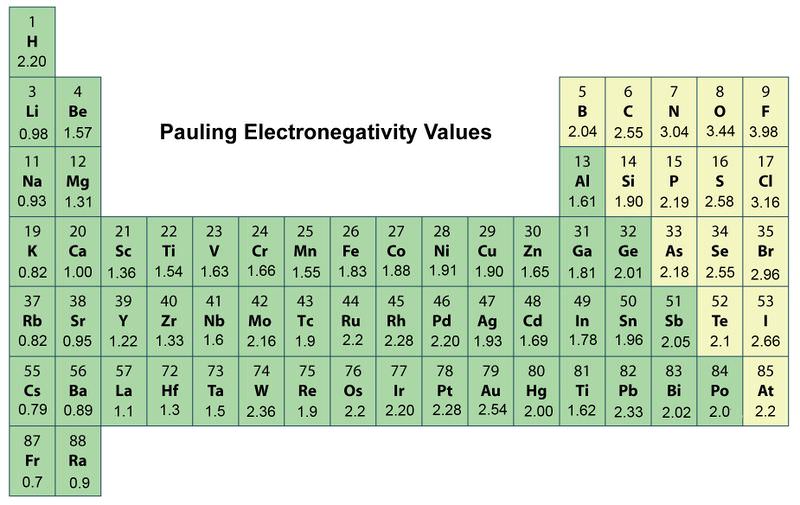
. So Electronegativity increases up and to the right describes the trends the best. --Electronegativity increases down and to the left. Electronegativity values generally increase from left to right across the periodic table.
A increases across a period and decreases down a group. As you go across a period the electronegativity increases. Electronegativity increases left to right and bottom to top.
--Electronegativity decreases up and to the left. Which of the. When we move from left to right in a period of the modern periodic table electronegativity increases.
Electronegativity decreases up and to the left. Trends in electronegativity across a period. Answer choices Helium on the upper right has the highest electronegativity and Francium on the lower left has the lowest electronegativity.
It sees a decreasing trend when you move down a group. Which statement describes the general trends in electronegativity and metallic properties. When you move from left to right it increases in the periodic table But when you move down the table electronegativity decreases.
Electronegativity usually rises from left to right. D decreases across a period and increases down a group. It doesnt have an electronegativity because it doesnt form bonds.
Which best describes the trends in electronegativity on the periodic table. For example the electronegativity trend across period 3 in the periodic table is depicted below. Which one of the following describes the correct trends in electronegativity in the periodic table.
Which statement describes the general trends in electronegativity and first ionization. Lithium 10 and Fluorine 40 in period 2. Which statement accurately describes a pattern in the.
Trends in electronegativity down a group. The electronegativity also increases up a group column of the periodic table. Electronegativities of the elements Na Al P and Cl follow a specific trend across the period.
Which statement best describes how electrons fill orbitals in the periodic table. While this is the basic definition of the electronegativity trend to truly understand it it would be helpful to. As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table.
--Electronegativity increases up and to the right. As you move down the periodic table elements have more protons and gain an electron energy shell so atoms become larger. The highest electronegativity value is for fluorine.
There electronegativity is around 30 and 40 while the left side has electronegativity ranging from 7 to 10. The electronegativity trend refers to a trend that can be seen across the periodic tableThis trend is seen as you move across the periodic table from left to right. The answer is Electronegativity increases up and to the right.
Which statement describes the trends of electronegativity values in the periodic table. A cation that has a larger radius. Which best describes trends in electronegativity on the periodic table.
We can see this with the help of a graph showing the trend in electronegativity in period 3 from sodium to chlorine. Electronegativity increases down and to the left. Which best describes trends in electronegativity on the periodic table General.
It shows how an atom can swiftly form a chemical bond. Electronegativities generally decrease from top to bottom of a group. As you go down a group electronegativity decreases.
What best describes the trends in electronegativity on the periodic table. The electronegativity increases while it decreases as you move down a group of elements. Lithium 10 and Francium 07 in Group I.
These related values display the same trend in the periodic table. The Periodic Table of the Elements is useful for revealing patterns and trends in the elements. Electronegativity decreases down and to the right.
B decreases across a period and decreases down a group. Values increase from left to right across a period and increase down a group. Light with any intensity above a certain frequency.
As you move across a row of the periodic table there are more protons and electrons but the electrons are held more closely to the nucleus so the overall size of the atom. Which best describes trends in electronegativity on the periodic. Electronegativity is the ability of an atom to pull electrons towards it.
A - The ionization energy decreases because the full s orbital shields the electron entering the p orbital. However there are exceptions to it at times. The chart shows electronegativities from sodium to chlorine - you have to ignore argon.
Periodic Trends in the Electronegativities of Elements. In this graph we have not shown argon as it does not react with elements to form bonds. What best describes the trends in electronegativity on the periodic table.
Trends in electronegativity across a period. The trends for electronegativity is that the value increases across the periods rows of the periodic table. Which statement best describes the name and location on the Periodic Table of the elements with the highest and lowest electronegativity respectively.
Electronegativity is higher on the right side of the periodic table such as the noble gasses. C increases across a period and increases down a group. Electronegativity increases up and to the right.
Which best describes the trends in electronegativity on the periodic table. To see more answers head over to College Study Guides.

Periodic Trends Made Easy Chemtalk

Periodic Trends In Electronegativity Ck 12 Foundation

Page 6 Periodic Trends Lewis Structures Polarity Imf And Vsepr Theory Chemstem In 2021 Vsepr Theory Chemistry Classroom Chemistry Basics
Comments
Post a Comment